On November 6, 2023, the Centers for Medicare and Medicaid Services (CMS) announced a proposed Rule that would permit Medicare Part D plan sponsors to substitute non-interchangeable biosimilars in place of the biologic medicines now used to treat many chronic conditions such as rheumatoid arthritis, Crohn’s disease and cancer. The policy change represents a stark departure from the perspectives of the U.S. medical community and patient advocacy organizations, a decade of state-level policymaking, and CMS’ recent assurances, warns the Alliance for Safe Biologic Medicines.
“Substitution of biosimilars by someone other than the prescribing physician is a controversial practice banned in many countries, including nearly all of Western Europe”, says ASBM Executive Director Michael Reilly, who served as Associate Deputy Secretary of Health and Services during the development and implementation of the Part D prescription drug benefit. “It is also opposed by the majority of physicians, in the U.S. and worldwide.”
A 2021 survey of 401 U.S physicians found that while 89% of U.S. prescribers have high confidence in the safety and efficacy of biosimilars, a majority (58%) oppose third-party switching of a patient’s biologic medicine for non-medical (e.g. cost, coverage) reasons- as would occur under the proposed CMS rule. Further, 69% consider it very important or critical that physicians, with their patient, make these treatment decisions.
“Treatment plans aren’t one-size fits all”, explains ASBM Chairman and practicing gastroenterologist Ralph McKibbin, MD, FACP, FACG, AGAF. “Patients often try several products over years before finding the one that best stabilizes their condition. As doctors and patients, we’re reluctant to switch a patient’s medicine unnecessarily and risk losing those gains.”
U.S. state laws nationwide permit pharmacy-level substitution of “interchangeable biosimilars”- those for which the manufacturer has provided additional safety and efficacy data to the FDA showing that a patient who’s been switched to the interchangeable product will have the same results as staying on the originator product. These laws were passed state by state over the past decade with the support of physician societies and patient advocacy organizations- contingent on assurances that only interchangeable biosimilars would ever be substituted without physician approval.
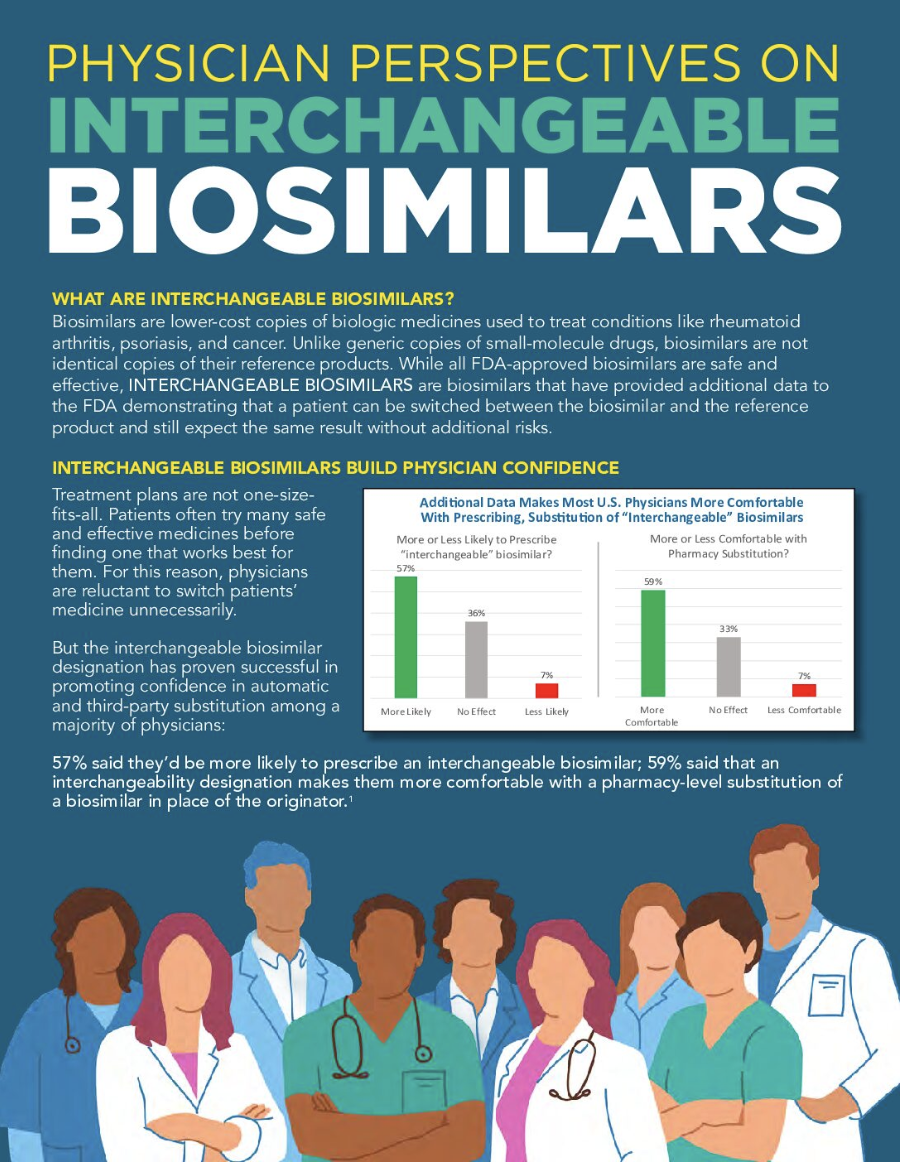
The additional data the interchangeable biosimilar carries dramatically boosts physician confidence: most (57%) said they would be more comfortable prescribing an interchangeable, and most (59%) are more comfortable with it being substituted in place of the prescribed originator product. Seven of the 44 FDA-approved biosimilars are interchangeable.
The dramatic change in policy proposed by CMS comes less than a year after a CMS Rule permitting Part D plan sponsors to substitute interchangeable biosimilars explicitly reassured the public it would not permit substitution of non-interchangeable biosimilars because they “have not met the requirements to support a demonstration of interchangeability.”
“Nothing has changed regarding non-interchangeable biosimilars since last year’s CMS Rule,” says Reilly. “Non-interchangeables still haven’t met the FDA data requirements for interchangeability and still shouldn’t be substituted by a third party without physician approval. This ill-considered move stands in stark contrast to the opinions of the medical community, the wishes of patients, a decade of substitution policymaking across 50 states, the substitution policies of most advanced nations, and CMS’ own assurances. We respectfully urge CMS to reconsider and withdraw this rule.”
For more information on interchangeable biosimilars, Reilly directs the public to several ASBM resources on the topic including a recent podcast, a letter to Congress supporting the standard, a video on physician perspectives, and an educational backgrounder.
ASBM is an organization of physician, patient advocates, pharmacists, and manufacturers of both biologic medicines and biosimilars. Since 2010, ASBM has worked to keep patients at the center of health policymaking. Learn more at www.safebiologics.org. Contact:media@safebiologics.org
View a PDF of this statement https://safebiologics.org/wp-content/uploads/2023/11/CMS-NICBS-STMT-FNL-1.pdfhere.