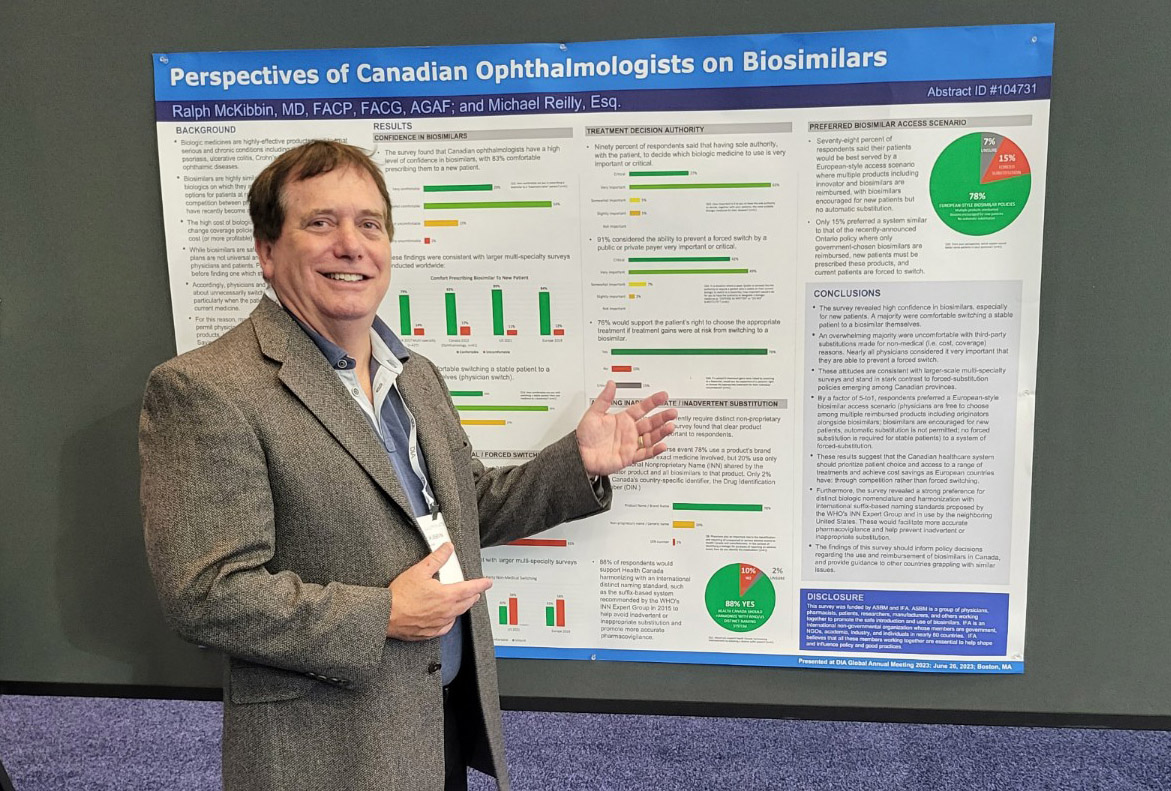
On June 26th, ASBM Chairman Ralph McKibbin presented a poster at the DIA Global Annual Meeting in Boston, MA. The poster was based on the findings from ASBM’s Canadian ophthalmologist survey and compared the findings with those of other physicians surveyed in recent years. The surveys examined physician attitudes toward biosimilar substitution practices, pharmacovigilance and nomenclature standards, and different biosimilar access scenarios.
View the poster For example, 81% of ophthalmologists were uncomfortable with a third-party payer (such as a government or private insurer) switching their patient’s biologic medicine for non-medical reasons (e.g. cost or coverage), as occurs under the government health plans of several provinces. 90% believe treatment decisions should rest with the physician and patient, and 91% believe they should be able to prevent a forced switch. Ophthalmologists are the latest group of physicians to raise concerns with the practice of forced switching- which has become increasingly common among Canadian provinces.
View Dr. McKibbin’s video walkthrough of the poster Prior ASBM surveys across many specialties revealed that 83% of Canadian, 82% of European, and 69% of U.S. physicians consider it very important or critical that the physician and patient control treatment decisions, including the decision to switch to a biosimilar. 79% of Canadian, 84% of European, and 67% of U.S. physicians considered the ability to prevent a substitution similarly important. View the poster here.
View Dr. McKibbin’s video walkthrough of the poster here.