March 2019 Newsletter
April 1, 2019
|
April 1, 2019
|
March 26, 2019
From March 22nd-24th, ASBM exhibited at the Annual Meeting and Expo of the American Pharmacists Association (APhA), held in Seattle, Washington.Founded in 1852, APhA is the largest association of pharmacists in the United States, with more than 62,000 practicing pharmacists, pharmaceutical scientists, student pharmacists, pharmacy technicians as members.

ASBM was represented at its booth by Advisory Board Chair Philip Schneider, past president of the American Society of Healthsystem Pharmacists (ASHP) and Advisory Board Member Ronald Jordan, Dean of the Chapman University College of Pharmacy and past president of APhA.
Dr. Schneider answered questions about biosimilar policy issues which affect pharmacy practice, including naming practices in the US and internationally. US biosimilar substitution policy, which varies by state, was also discussed.

Educational videos targeted at pharmacists were also shown at the ASBM booth during the three-day exhibition. These included a video featuring Schneider and Jordan, and ASBM’s recently-released pharmacist videos on naming, interchangeability and substitution, and non-medical switching were also shown.
March 12, 2019
ASBM commends the decision by FDA to apply distinguishable suffixes to all biologics, including interchangeable biosimilars, going forward. FDA’s decision puts in place a protocol for a safe future, when there are many more biologics, biosimilars and interchangeable biosimilars. This policy ensures that patients and health care providers can distinguish between products when that is important for a patient’s care or to report adverse events.
We understand FDA’s thoughtful solution to the difficult conundrum of products approved prior to adoption of the suffix policy. FDA will not change the nonproprietary names of already approved products in order to avoid confusion that could result. For these long-standing products, the brand name will be the distinguishing element and require more vigilance and effort by prescribers. This exception to the suffix protocol will ultimately play a role in the traceability of a limited number of biologics given that most products will not have biosimilars due to the challenges of biosimilar development.
March 7, 2019
On March 6th in Ottawa, Ontario, ASBM hosted the third in a series of meetings between health regulators and other stakeholders from around the globe to discuss the international harmonization of biologic nomenclature and the importance of distinguishable naming. Representatives from Health Canada, the FDA, and WHO participated.

The meeting began with opening remarks by ASBM Executive Director Michael Reilly thanking the participants. He emphasized the shared support for global harmonization and for WHO leadership in that effort. Participants then introduced themselves; they included:
ASBM Advisory Board Chair Philip Schneider then presented a recap of the first two meetings, which were held in April and July of last year in Washington, DC. In these meetings, a strong consensus emerged on the importance of distinct naming and in favor of international harmonization. Health Canada in particular was a leading advocate of both.
Schneider then recounted several developments in the naming discussion that have occurred since the July meeting. First was the publication of the Scientific American whitepaper based on the April meeting, which may be read here. Second was ASBM’s participation in the 67th INN Consultation on October 13, 2018. Third was the publication of an article in the GaBI Journal emphasizing the benefits of the WHO’s Biological Qualifier (BQ) proposal for countries in the Middle East and North Africa region without robust pharmacovigilance systems of their own. The BQ would assign a 4-letter suffix to all biologics and biosimilars sharing an INN.
Dr. Schneider also shared objections to distinct naming- in particular to the WHO’s BQ proposal- that have been raised over the years, including that the system would be redundant, or might impede access, and proceeded to address them. For example, while advanced countries in Europe have robust pharmacovigilance systems, many developing nations do not. The Ministry of Health in the United Arab Emirates, Schneider noted, has expressed its support for the WHO’s BQ proposal, as have physicians in Latin America, 94% of whom consider it helpful in ensuring their patients receive the correct medicine.
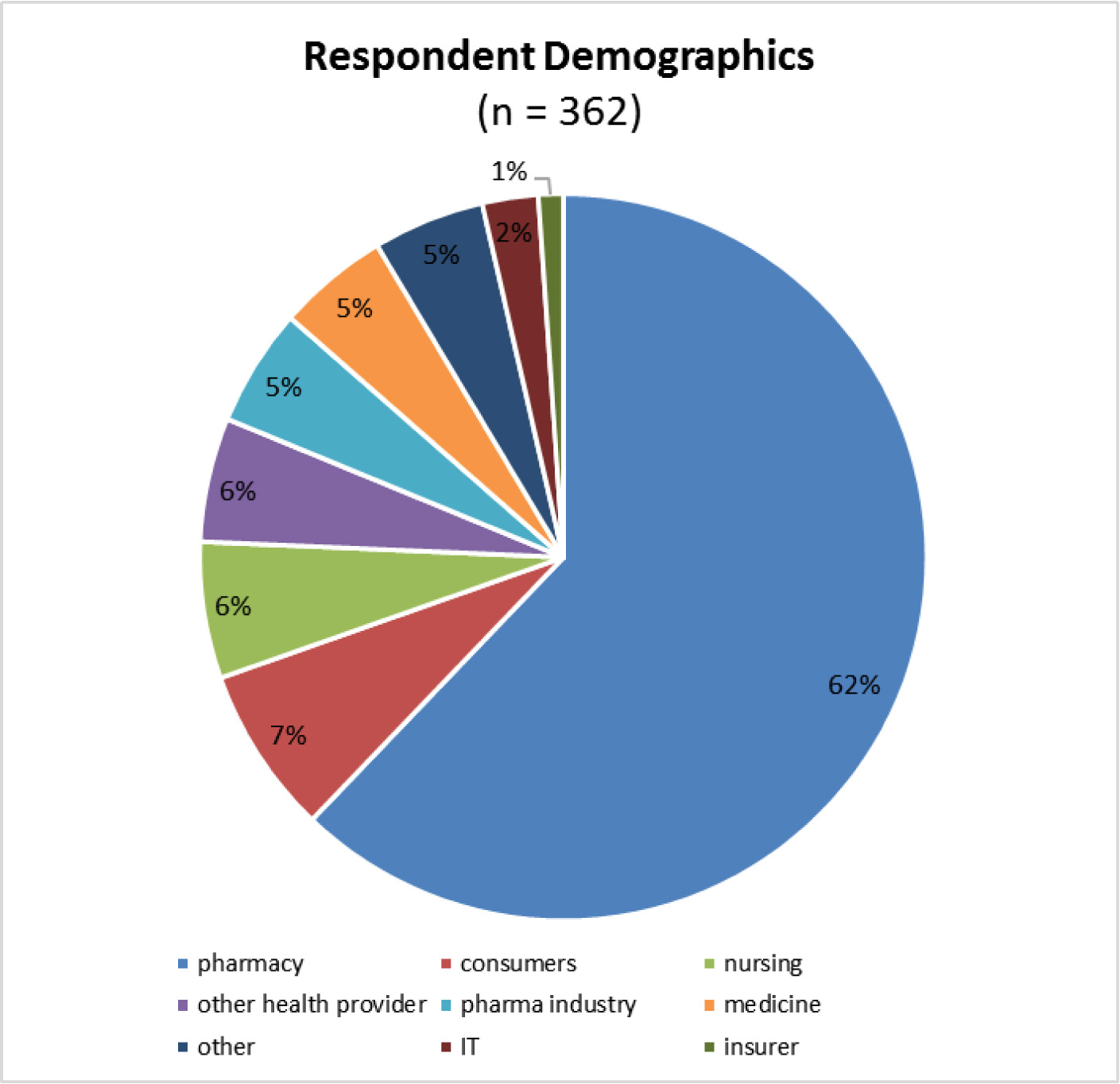
As Dr. Schneider observed, this consultation and the resulting policy fails to adequately consider not only the perspectives of patients, but that of physicians.
 For example, in a 2017 survey of 403 Canadian prescribers of biologics, ASBM found:
For example, in a 2017 survey of 403 Canadian prescribers of biologics, ASBM found:

FDA official Lubna Merchant, who attended the first two meetings, joined the meeting via teleconference.




 Dr. Balocco also expressed hope that Canada’s decision would further underscore the urgent need for the WHO to take a leadership role on biologic naming and implement the BQ standard.
Dr. Balocco also expressed hope that Canada’s decision would further underscore the urgent need for the WHO to take a leadership role on biologic naming and implement the BQ standard.

March 2, 2019
On January 7th, ASBM Chair Madelaine Feldman, MD FACR was interviewed by the Center for Biosimilars at a forum comprised pharmacists, clinicians, and representatives from biosimilar development companies. The topic was education on biosimilars. Dr Gillian Woollett of Avalere Health moderated the discussion, which was recorded at the group’s studio in New Jersey.
Clips from the interview may be viewed here throughout the month of March.
Below is the first clip, in which Dr. Feldman discusses the concept of interchangeability, post-market challenges to biosimilar uptake, the importance of physician education on biosimilars.
March 2, 2019
On January 7th, ASBM Chair Madelaine Feldman, MD FACR was interviewed by the Center for Biosimilars at a forum comprised pharmacists, clinicians, and representatives from biosimilar development companies. The topic was education on biosimilars. Dr Gillian Woollett of Avalere Health moderated the discussion, which was recorded at the group’s studio in New Jersey.
Clips from the interview may be viewed here throughout the month of March.
Below is the first clip, in which Dr. Feldman discusses the concept of interchangeability, post-market challenges to biosimilar uptake, the importance of physician education on biosimilars.
February 1, 2019
|
January 16, 2019
On January 10, 2019, the Washington Post published an article “Patients stuck in corporate fight against generic drugs.” I spoke extensively with the author of the article and also suggested that he speak with the Chair of the Alliance for Safe Biologic Medicines (ASBM) international advisory board Dr. Philip Schneider. Unfortunately, the article did not represent the information that was shared by me and Dr. Schneider about the work of ASBM. Instead it attempted to portray ASBM as an organization seeking to “create confusion about the safety and effectiveness of unbranded biologic drugs” which is an assertion that cannot be credibly made in light of ASBM’s record.
Since 2013, ASBM has been involved in efforts to pass state legislation that is intended to increase access to biosimilars by allowing pharmacists to automatically substitute interchangeable biosimilars once approved by the FDA without consulting with the prescribing physician–provided the physician is notified within 72 hours. This legislation is consistent with the position of the FDA and removes the prior authorization or notification barrier that can delay access to biosimilars. We have provided testimony either in written form or in-person in each of the 45 states where these laws have been passed.
ASBM has, from its inception in 2010, been focused on serving as a resource for regulators and policymakers in order to help establish a robust biosimilars program that will ultimately increase access and reduce the cost of medicine for patients and governments worldwide. I have served as the Executive Director of ASBM since it was formed. Our focus on affirmatively establishing – not thwarting – a pathway for biosimilars, including substitution when appropriate – was built on my experience in government, including three tours at the U.S. Department of Health and Human Services (HHS) under the 41st, 42nd and 43rd Presidents. I served my final tour at HHS working in the Office of the Secretary from 2002 to 2008 and know from firsthand experience how important thoughtful participation by informed experts is to sound public policy.
Since 2010, ASBM has participated in more than 50 meetings with regulators worldwide to discuss policy issues and challenges around biosimilars. In every instance, ASBM has begun with the underlying and fundamental assertion that biosimilars are an important tool in the attempt to increase access and reduce the cost of medicines. We have provided in-person testimony to the FDA on at least 15 occasions during that time. I have participated on panels discussing biosimilar policy with regulators in Berlin, Brussels, Canberra, Dublin, Geneva, Madrid, Ottawa, Paris, and Rome and met directly with senior government officials from the European Commission (Brussels), European Medicines Agency (EMA), FDA, Health Canada, Italian & Spanish Ministries of Health, Therapeutic Goods Administration (TGA) and World Health Organization (WHO). We have conducted 14 physician surveys in 12 countries and shared the results of those surveys directly with the regulators in the countries surveyed. All of the surveys are publicly available on our website www.safebiologics.org/surveys.
ASBM will continue to serve as a resource for policymakers and regulators in the U.S. and across the globe as they attempt to build a robust and sustained biosimilars program that will benefit patients worldwide.
Michael Reilly
Executive Director, ASBM
January 14, 2019
by Philip Schneider, MS FASHP FFIP
Advisory Board Chair, Alliance for Safe Biologic Medicines
This is to clarify an irresponsible misrepresentation by Christopher Rowland in his article “Patients Stuck In Corporate Fight Against Generic Drugs,” published in the January 9, 2019 edition of the Washington Post.
My comments that were misinterpreted in this article arose from a discussion about the importance of duly considering safety and effectiveness, in addition to cost, when making decisions about the choice of drug therapy. My mention of thalidomide was in reference to a seminal event that made ensuring the safety of drugs and other medical products a priority for the FDA and to show how the FDA has ably fulfilled this mission in the ensuing decades. In no way was I linking biosimilars to the thalidomide experience. The basic underpinning of every presentation I have ever given, comments I have submitted or testimony I have provided is that all biosimilars are to be considered “safe and effective” once approved by the FDA.
By way of background, I was interviewed as chairman of the international advisory board of the Alliance for Safe Biologic Medicines (ASBM). In this capacity, I represent an organization that since 2010 has promoted biosimilars as a strategy to increase access to new breakthrough biologic therapies.
I bring to my role with ASBM more than forty years of experience in academic health sciences centers as both a practitioner and faculty member; I have never been an employee of the pharmaceutical industry. My comments regarding the safety of biosimilars were taken out of context and wrongly portrayed. I have testified on behalf of ASBM in many states in support of legislation that authorizes pharmacists to automatically substitute less expensive interchangeable biosimilars approved by the FDA; legislation that has now been passed in 45 of 50 states in the US.
I have also testified on eight occasions to the World Health Organization in support of their Biologic Qualifier program that assigns distinguishable non-proprietary names to biosimilars to improve confidence in their use among prescribers, pharmacists, and patients to speed uptake. I have conducted many continuing education programs for health care professionals promoting the safe use of biosimilars; something quite inconsistent with the impression left in the Washington Post article.
Let me be clear: I support the availability and use of biosimilars. By taking one of my comments out of context, the article misleads readers into believing that I oppose them. I am writing this to correct this irresponsible reporting by the Post.
January 14, 2019
by Philip Schneider, MS FASHP FFIP
Advisory Board Chair, Alliance for Safe Biologic Medicines
This is to clarify an irresponsible misrepresentation by Christopher Rowland in his article “Patients Stuck In Corporate Fight Against Generic Drugs,” published in the January 9, 2019 edition of the Washington Post.
My comments that were misinterpreted in this article arose from a discussion about the importance of duly considering safety and effectiveness, in addition to cost, when making decisions about the choice of drug therapy. My mention of thalidomide was in reference to a seminal event that made ensuring the safety of drugs and other medical products a priority for the FDA and to show how the FDA has ably fulfilled this mission in the ensuing decades. In no way was I linking biosimilars to the thalidomide experience. The basic underpinning of every presentation I have ever given, comments I have submitted or testimony I have provided is that all biosimilars are to be considered “safe and effective” once approved by the FDA.
By way of background, I was interviewed as chairman of the international advisory board of the Alliance for Safe Biologic Medicines (ASBM). In this capacity, I represent an organization that since 2010 has promoted biosimilars as a strategy to increase access to new breakthrough biologic therapies.
I bring to my role with ASBM more than forty years of experience in academic health sciences centers as both a practitioner and faculty member; I have never been an employee of the pharmaceutical industry. My comments regarding the safety of biosimilars were taken out of context and wrongly portrayed. I have testified on behalf of ASBM in many states in support of legislation that authorizes pharmacists to automatically substitute less expensive interchangeable biosimilars approved by the FDA; legislation that has now been passed in 45 of 50 states in the US.
I have also testified on eight occasions to the World Health Organization in support of their Biologic Qualifier program that assigns distinguishable non-proprietary names to biosimilars to improve confidence in their use among prescribers, pharmacists, and patients to speed uptake. I have conducted many continuing education programs for health care professionals promoting the safe use of biosimilars; something quite inconsistent with the impression left in the Washington Post article.
Let me be clear: I support the availability and use of biosimilars. By taking one of my comments out of context, the article misleads readers into believing that I oppose them. I am writing this to correct this irresponsible reporting by the Post.