Lower med standards endangers health
Altoona Mirror
Opinion
Oct 2, 2024
Dr. Ralph McKibbin
As a practicing physician in Pennsylvania, I firmly believe that the relationship between a doctor and their patient should guide every treatment decision.
When physicians recommend treatments, we rely on years of training, clinical evidence, and the unique medical history of each patient.
Patients should have the confidence to make decisions about their care in partnership with their doctor, without the fear that insurance companies or pharmacies will override those choices for non-medical reasons such as increasing profitability.
Unfortunately, a proposed policy change threatens to undermine this relationship and could disrupt the treatment stability of patients across Pennsylvania.
Biologic medications are commonly prescribed to treat conditions like cancer, arthritis and Crohn’s disease — serious chronic conditions that require careful management. “Biosimilars,” which are designed to mimic these biologic medications, help create competition and lower costs.
While these drugs are safe and effective, they are not exact copies of the original biologics. Because of this, insurers or pharmacies can’t simply switch my patients to a biosimilar without my approval, unlike with generic drugs.
However, the FDA does allow some biosimilars to be designated as “interchangeable,” meaning that a pharmacist could substitute the biosimilar for the original biologic without needing approval from the prescribing doctor.
To receive this designation, manufacturers must submit additional data, which sometimes includes patient studies to prove that safety and effectiveness remain the same — even if a patient is switched between the original and biosimilar multiple times.
While nearly 9 in 10 physicians trust the safety of biosimilars, the majority (69%) believe that the decision of which medication to use should be made by patients and their doctors — not insurers or pharmacies.
This is because treatment is never one-size-fits-all. Many of my patients have had to try several medications before finding the one that best stabilizes their condition. Unnecessary or inappropriate switching risks disrupting this hard-earned stability.
That’s why 59% of doctors are more comfortable with biosimilar substitutions when the FDA designates them as interchangeable — it assures us that the switch won’t compromise our patients’ health.
Congress, including Pennsylvania Sen. Bob Casey, is considering whether or not to remove these important safeguards.
The Biosimilar Red Tape Elimination Act would classify all biosimilars as interchangeable, allowing insurance companies to switch patients to any biosimilar without prior physician approval — even if it hasn’t been demonstrated to the FDA that such switching won’t jeopardize treatment stability.
The bill would also effectively strip the FDA of its ability to request studies on switching, weakening the standards that currently protect patients.
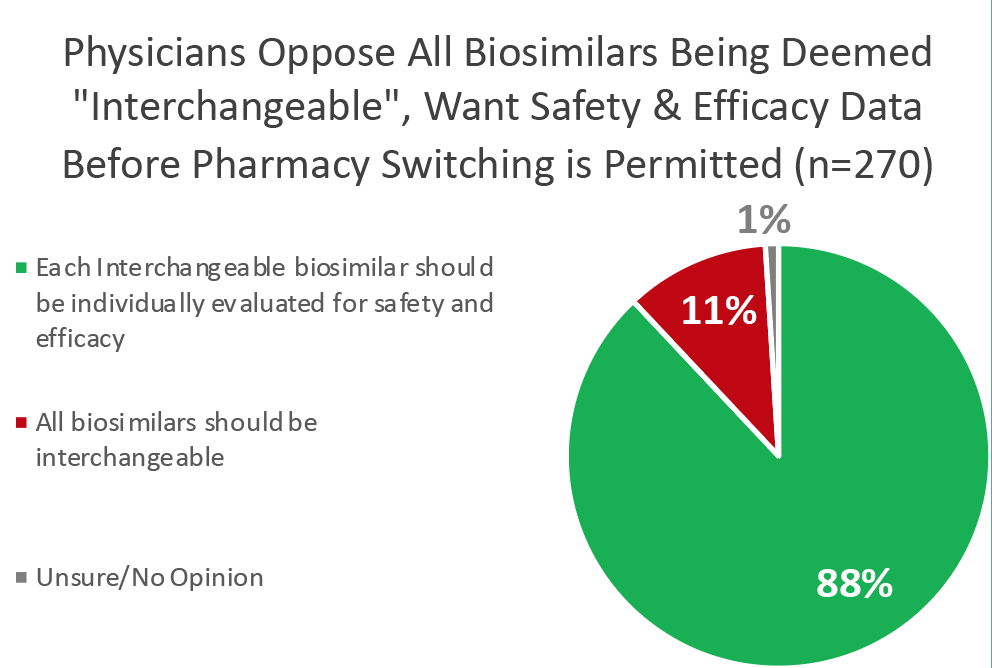
It’s no surprise that doctors across the country overwhelmingly oppose these changes. A recent survey of 270 U.S. physicians who prescribe biologics showed that only 11% support classifying all biosimilars as interchangeable, and 88% believe that switching studies increase their confidence of safely switching their patients to biosimilars.
As physicians, our primary concern is our patients’ health and safety.
It is Congress’s responsibility to maintain the rigorous standards for biosimilar interchangeability that have protected patients, earned doctors’ trust, and saved our healthcare system $24 billion.
Failing to do so would put patient health at risk and erode physician confidence in these vital treatments.
Dr. Ralph McKibbin, MD, FACP, FACG, AGAF, is the chairman of the Alliance for Safe Biologic Medicines, and a Pennsylvania-based gastroenterologist. He serves as Clinical Assistant Professor of Gastroenterology at Duquesne University College of Osteopathic Medicine; and Director at Pennsylvania Society of Gastroenterology.
 From the Fact Sheet: U.S. Physicians Strongly Oppose Pharmacy Substitution of Non-Interchangeable Biosimilars
From the Fact Sheet: U.S. Physicians Strongly Oppose Pharmacy Substitution of Non-Interchangeable Biosimilars