
Who We Are
The Alliance for Safe Biologic Medicines is an organization of patients, physicians, pharmacists, biotechnology companies that develop innovative and biosimilar medicines and others, who are working together to ensure that patient safety is at the forefront of the biosimilars policy discussion. It is the mission of the Alliance to serve as an authoritative resource center of information for the public, medical community, the FDA and other state and federal policymakers during the implementation of the biosimilars approval pathway and beyond.
Our Perspective
Biologics are advanced prescription drugs to treat cancer, rheumatoid arthritis and other debilitating diseases. In November 2010 the Food and Drug Administration began consultation with patient groups, physicians and industry on how to approve the first copies of these drugs, known as follow-on biologics or biosimilars. As the FDA moves forward in implementing this pathway, the Alliance for Safe Biologic Medicines will work to ensure patient safety remains the priority.
| SAVE THE DATE: ASBM and GaBI to Present Webinar on the Biosimilar Red Tape Elimination Act October 31st On October 31st, ASBM and the Generics and Biosimilar Initiative will present a webinar focusing Senate Bill 2305, the Biosimilar Red Tape Elimination Act. Health policy experts will review current U.S. biosimilar approval and substitution policies, discuss the rationale behind them, and examine the potential consequences of the proposed changes. Healthcare practitioners and patient advocacy representatives will also share their concerns about the Biosimilar Red Tape Elimination Act’s effects on physican confidence in biosimilars and its potential to jeopardize treatment stability for patients nationwide. More details and the program agenda will be made available soon. Read more about physician concerns with S.2305 here. |
| Dr. McKibbin Op-ed: Lowering the Interchangeable Biosimilar Standard Endangers Patient Health On October 3rd, an op-ed by ASBM Chairman Ralph McKibbin MD ran in the Pennsylvania-based Altoona Mirror. In the article, Dr. McKibbin highlights physician concerns with Senate Bill 2035, the “Biosimilar Red Tape Elimination Act”, under consideration by the Senate Committee on Health, Education, Labor, and Pensions (Senate HELP). The U.S. physicians who prescribe biologic medicines, however, do not support the bill’s provisions, McKibbin explains: The Biosimilar Red Tape Elimination Act would classify all biosimilars as interchangeable, allowing insurance companies to switch patients to any biosimilar without prior physician approval — even if it hasn’t been demonstrated to the FDA that such switching won’t jeopardize treatment stability. The bill would also effectively strip the FDA of its ability to request studies on switching, weakening the standards that currently protect patients. It’s no surprise that doctors across the country overwhelmingly oppose these changes. A recent survey of 270 U.S. physicians who prescribe biologics found that only 11% support classifying all biosimilars as interchangeable, and 88% believe that switching studies increase their confidence of safely switching their patients to biosimilars. As physicians, our primary concern is our patients’ health and safety.It is Congress’s responsibility to maintain the rigorous standards for biosimilar interchangeability that have protected patients, earned doctors’ trust, and saved our healthcare system $24 billion. Read the full op-ed here. |
| ASBM’s Michael Reilly: Congress Should Maintain Current FDA Biosimilars Standards On September 25th, RealClearHealth published an op-ed by ASBM Executive Director Michael Reilly, discussing widespread physician opposition to key components of S.2305, “The Biosimilar Red Tape Elimination Act”, which would declare all biosimilars interchangeable and thus, substitutable at the pharmacy level by insurance companies and PBMs. State medical societies nationwide support the laws now permitting the substitution of interchangeable biosimilars. However, that support was conditional on assurances from policymakers that third-party substitution would be limited only to interchangeable biosimilars backed by additional safety and efficacy data. But the safeguards physicians count on may soon be removed. This week, the Senate HELP Committee, which includes my own senator, Tim Kaine of Virginia, will vote on a bill that would classify all biosimilars as interchangeable. It would permit insurance companies to switch patients to any biosimilar as if they were generics, removing prior physician approval, in absence of now-required data demonstrating that switching won’t reduce safety and efficacy. The Biosimilar Red Tape Elimination Act would also remove the FDA’s ability to ask for switching studies when appropriate, dangerously lowering current safety standards. If this bill becomes law, it could negatively impact treatment stability for millions of patients. Reilly cited the findings of ASBM’s recent survey of 270 prescribers of biologic medicines. That survey revealed that only 11% support declaring all biosimilars interchangeable. 88% said switching studies increased their confidence in safe biosimilar substitution. The survey results come at a time when the interchangeable biosimilar standard faces several policy challenges. In August, ASBM shared the findings with the FDA as part of a public comment period on Draft Guidance that would de-emphasize switching studies. Read the full op-ed here. Read the U.S. physician survey here and the press release about its findings here. Read more about S.2305 here. |
| ASBM Chairman Discusses Interchangeable Biosimilar Policy at Washington, DC Policy Forums On September 23rd and 24th, ASBM Chairman Ralph McKibbin, MD FACP FACG AGAF visited Washington, DC to participate in two policy forums at which he shared physician perspectives on biosimilars policy. The first event was a 2024 Fall Policy Forum hosted on Capitol Hill by the Digestive Disease National Coalition (DDNC). The meeting was attended by Hill staff and key lawmakers who work on health policy. Attendees included Representatives Jim McGovern (D-MA) and Buddy Carter (R-GA). Representative Carter is a pharmacist and member of the House Energy & Commerce Committee’s Health Subcommittee. Dr. McKibbin used the meeting as an opportunity to share results of ASBM’s recent physician survey, showing strong opposition to S.2305, “The Biosimilar Red Tape Elimination Act” (see above articles) which would declare all biosimilars interchangeable and thus, substitutable at the pharmacy level by insurance companies and PBMs. The following day, Dr. McKibbin participated in a panel discussion entitled “the Changing Landscape of Biologics”, as part of the Alliance for Patient Access’ (AfPA’s) 9th Annual Policy & Advocacy Summit. McKibbin provided the physician perspective on a variety of topics including interchangeable biosimilar and substitution policy, non-medical switching, and the continued need for physician reimbursement and Pharmacy Benefit Manager (PBM) reforms. View Dr. McKibbin’s panel discussion here. |
ASBM Fact Sheet: Physicians Oppose Senate Bill S.2305 That Would Make ALL Biosimilars Interchangeable, Remove FDA Ability to Consider Switching Studies In September, ASBM released an updated fact sheet on Senate Bill S. 2305 “the Biosimilar Red Tape Elimination Act”, sponsored by Sen. Mike Lee (R-UT), showing how physicians overwhelmingly oppose its provisions. 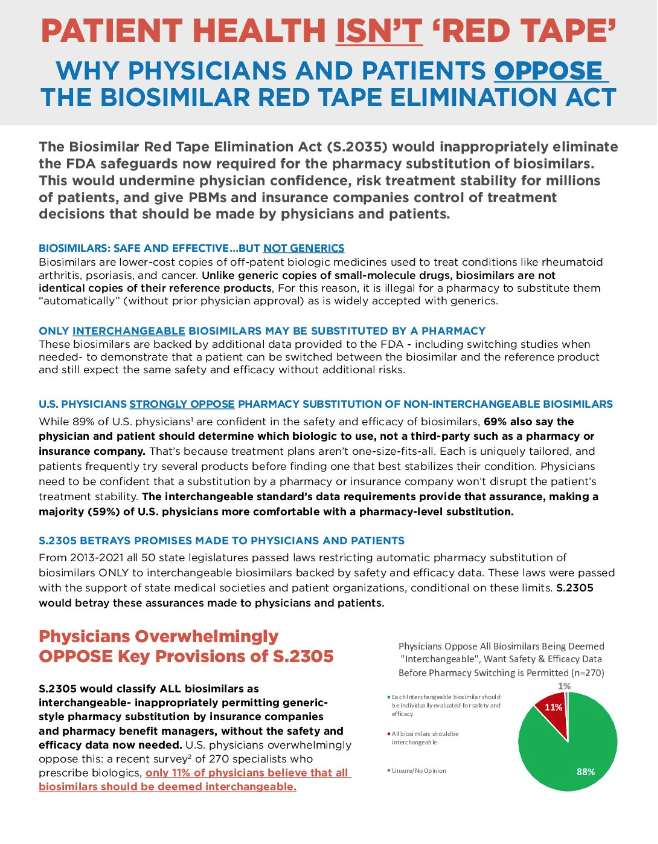 From the Fact Sheet: U.S. Physicians Strongly Oppose Pharmacy Substitution of Non-Interchangeable Biosimilars From the Fact Sheet: U.S. Physicians Strongly Oppose Pharmacy Substitution of Non-Interchangeable BiosimilarsWhile 89% of U.S. physicians are confident in the safety and efficacy of biosimilars, 69% also say the physician and patient should determine which biologic to use, not a third-party such as a pharmacy or insurance company. That’s because treatment plans aren’t one-size-fits-all. Each is uniquely tailored, and patients frequently try several products before finding one that best stabilizes their condition. Physicians need to be confident that a substitution by a pharmacy or insurance company won’t disrupt the patient’s treatment stability. The interchangeable standard’s data requirements provide that assurance, making a majority (59%) of U.S. physicians more comfortable with a pharmacy-level substitution. From 2013-2021 all 50 state legislatures passed laws restricting automatic pharmacy substitution of biosimilars ONLY to interchangeable biosimilars. These laws were passed with the support of state medical societies and patient organizations, conditional on these limits. S.2305 would classify ALL biosimilars as interchangeable-inappropriately permitting generic-style pharmacy substitution by insurance companies and pharmacy benefit managers, without the safety and efficacy data now needed. This would jeopardize treatment stability for millions of patients and betray the assurances made to physicians and patients nationwide that only biosimilars which had provided additional safety and efficacy data would ever be substituted without physician approval. It would also restrict what data FDA can ask for to approve an interchangeable biosimilar, requiring the HHS Secretary to hold a private briefing with the chairs and ranking members of the Senate HELP and House Energy and Commerce Committees to justify asking for a study demonstrating switching won’t reduce safety or efficacy in patients. Read the full Fact Sheet here. |
| ASBM Letter to US Senate Details Overwhelming Physician Opposition to “Biosimilar Red Tape Elimination Act” On September 6, ASBM sent a letter to Senator Mike Lee (R-UT), sponsor of Senate Bill 2305 (S.2305), the “Biosimilar Red Tape Act”. The letter was also sent to cosponsors of the bill, including Sen. Mike Braun, (R-IN), Sen Ben Ray Lujan (D-NM), Sen. Rand Paul (R-KY), and Sen. JD Vance (R-OH). The letter details the opposition among U.S. physician opposition to key components of S. 2305. These include a provision that the FDA designate all biosimilars as interchangeable (and thus substitutable like generics- without physician involvement- at the pharmacy level by third parties such as insurance companies and pharmacy benefit managers (PBMs). The bill also removes the FDA’s authority to consider switching studies when making a determination that safety and efficacy do not diminish following a biosimilar substitution. From the letter: The letter shared survey data from ASBM’s August survey of 270 physicians, which showed strong opposition to key provisions of S.2305. For example, only 11% of physicians supported making all biosimilars interchangeable, as the bill would. In addition, 88% said that switching studies- showing no loss of efficacy or safety- increased their confidence in an interchangeable biosimilar being substituted. S. 2035, however, would remove the FDA’s ability to consider such studies during biosimilar approval. Read the letter to Sen. Lee and the bill’s cosponsors here. Read more about S. 2035 here. Read ASBM’s physician survey here and the press release about its findings here. |
| ASBM/Ohio State University College of Pharmacy Course Examines Impact of Lowering Interchangeable Biosimilar Standards, IRA On September 30, Philip Schneider, ASBM Advisory Board Chair, taught a 2-hour class at the University of Colorado-Boulder’s College of Pharmacy entitled “Biosimilar Medicines”. The course examined how recent and proposed biosimilar policy changes may impact pharmacy practice within the biopharmaceutical industry. The module examined three current policy issues related to biosimilars:The likely negative impact of the Inflation Reduction Act’s price-setting on biosimilar development and commercialization; Senate Bill 2035 (the Biosimilar Red Tape Elimination Act) which would lower the requirements for interchangeable biosimilars and/or declare all biosimilars interchangeable and thus pharmacy-substitutable; and Various efforts to reform Pharmacy Benefit Manager (PBM) utilization management and formulary design practices. Pharmacy students were given basic information about each proposal, and then asked to research further and discuss challenges each policy might pose to increasing patient access to safe and affordable therapies. In February 2025, Schneider will offer a version of the course at Ohio State University’s College of Pharmacy, where he is a professor of pharmacy. ASBM and the OSU College of Pharmacy recently collaborated on a 7-part comprehensive series on biosimilars, led by Professor Schneider and featuring ASBM Chairman Ralph McKibbin, MD; Immediate Past Chair Madelaine Feldman, MD, and ASBM Steering Committee Member Andrew Spiegel of the Global Colon Cancer Association. The ACPE-accredited course is worth 7 hours of continuing education credit and is open to all pharmacists nationwide and may be accessed here. |
| ASBM and GaBI Present Webinar on the Biosimilar Red Tape Elimination Act On October 31st, ASBM and the Generics and Biosimilar Initiative presented a webinar focusing Senate Bill 2305 (S.2305) the Biosimilar Red Tape Elimination Act. Currently, state laws nationwide permit only “interchangeable” biosimilars to be automatically substituted by insurers of pharmacy-benefit managers (PBMs), the way generics may be substituted- without physician approval. Whil safe and effective, unlike generics biosimilar are not identical to their reference products. To earn the interchangeable designation, manufacturers of these biosimilars must demonstrate to the FDA that switching a patient will not reduce safety or efficacy of treatment, through additional data that sometimes including switching studies. The bill would for the first time permit widespread and unrestricted pharmacy-level substitution of all biosimilars, inappropriate treating them as generics for substitution purposes. During the webinar, ASBM Executive Director Michael Reilly and Advisory Board Chair Philip Schneider reviewed current U.S. biosimilar approval and substitution policies, discussed the rationale behind them, and examined the potential consequences of the proposed changes. Reilly, who recently co-authored a whitepaper about how misinformation is distorting U.S. interchangeable biosimilar policy, highlighted multiple misconceptions underpinning the bill, performing a “fact check” on several inaccuracies in a press release announcing its introduction. For example, the bill’s press release stated that biosimilars are equivalent to generics and that switching studies are required for approval as interchangeable. In reality, the FDA has said that “biosimilars are not generics and important difference exist between them” and that they “were able to approve the majority of interchangeable biosimilars without clinical switching studies.” In addition, the press release stated that the European Medicines Agency (EMA) determined that switching studies are not necessary for interchangeability, inaccurately suggesting that S.2305 would align the U.S. with European policy. In reality, the EMA has said its use of the term refers to substitution of biosimilars by the prescribing physician, not automatic pharmacy-level substitution of biosimilars, a practice banned in much of Western Europe and not within the remit of the EMA. ASBM Chairman Ralph McKibbin MD shared concerns that physicians have with the Biosimilar Red Tape Elimination Act. A recent survey of 270 U.S. physicians revealed only 11% support all biosimilars being deemed interchangeable. ASBM Steering Committee Member Andrew Speigel, Executive Director of the Global Colon Cancer Association, shared patient concerns about the Bill. In particular, Spiegel stressed the potential of the unprecendented large-scale third-party substitution the bill would unleash to jeopardize treatment stability for patients nationwide. More details and the program agenda will be made available soon. View the webinar here. |
ASBM Presents at 79th WHO INN Consultation
On October 22nd, ASBM presented to the World Health Organization International Nonpropretary Names (INN) Expert Group at the 79th Consultation on International Non-proprietary Names for Pharmaceutical Substances (INN), held in Geneva, Switzerland.
ASBM was represented at the session by Executive Director Michael Reilly, Esq., and Advisory Board Chair Philip Schneider, MS, FASHP. The proceedings of the INN Consultation are bound by confidentiality pending the publication of the Executive Summary by the WHO.
However, the Executive Summary from the 78th INN Consulation (held March 18, 2024 and at which ASBM also presented) lays out the benefits of a distinct global nomenclature standard, and highlights the ongoing support for such a standard, both from ASBM as well as from regulators:
The ASBM intends to defend the role of clinical data in biosimilar approval and this trend toward deemphasis of clinical data in biosimilar approval also makes strong post-marketing pharmacovigilance all the more important.
The benefits of a WHO led distinct nomenclature standard remain clear and would benefit the least economically-developed countries of the world. The meeting was reminded of previous ASBM studies that show strong support for distinct naming including regulators in large and small countries. Some regulators have adopted their own specific naming system and some who initially supported the BQ have initiated their own system would be happy to adopt a WHO global standard.
In summary, as the INN Group still supports its recommendation for distinct suffixes, as strong support for distinct naming remains amongst regulators and as lead regulators push for reduced emphasis on clinical trials in biosimilar approvals and stronger biologic pharmacovigilance becomes increasingly important, ASBM is determined to advance the proposal by working with the WHO and other National Regulatory Authorities worldwide.
Read the full Executive Summary of the 78th INN Consultation here.
ASBM Presents at 79th WHO INN Consultation
On October 22nd, ASBM presented to the World Health Organization International Nonpropretary Names (INN) Expert Group at the 79th Consultation on International Non-proprietary Names for Pharmaceutical Substances (INN), held in Geneva, Switzerland.
ASBM was represented at the session by Executive Director Michael Reilly, Esq., and Advisory Board Chair Philip Schneider, MS, FASHP. The proceedings of the INN Consultation are bound by confidentiality pending the publication of the Executive Summary by the WHO.
However, the Executive Summary from the 78th INN Consulation (held March 18, 2024 and at which ASBM also presented) lays out the benefits of a distinct global nomenclature standard, and highlights the ongoing support for such a standard, both from ASBM as well as from regulators:
The ASBM intends to defend the role of clinical data in biosimilar approval and this trend toward deemphasis of clinical data in biosimilar approval also makes strong post-marketing pharmacovigilance all the more important.
The benefits of a WHO led distinct nomenclature standard remain clear and would benefit the least economically-developed countries of the world. The meeting was reminded of previous ASBM studies that show strong support for distinct naming including regulators in large and small countries. Some regulators have adopted their own specific naming system and some who initially supported the BQ have initiated their own system would be happy to adopt a WHO global standard.
In summary, as the INN Group still supports its recommendation for distinct suffixes, as strong support for distinct naming remains amongst regulators and as lead regulators push for reduced emphasis on clinical trials in biosimilar approvals and stronger biologic pharmacovigilance becomes increasingly important, ASBM is determined to advance the proposal by working with the WHO and other National Regulatory Authorities worldwide.
Read the full Executive Summary of the 78th INN Consultation here.
