ARLINGTON, Va., June 6, 2019 /PRNewswire/ — On May 27th, the Government of British Columbia (B.C.) announced a policy that will forcibly switch thousands of patients with serious, chronic conditions from their current biologic medicines to lower-cost “biosimilar” treatments, effective November 25th, 2019. The roughly 23,000 patients who will be affected include those with rheumatoid arthritis, plaque psoriasis, psoriatic arthritis, diabetes, Crohn’s Disease, and ulcerative colitis. The announcement raises concerns among patients and physicians, according to the Alliance for Safe Biologic Medicines (ASBM), an international organization of patient advocates, physicians, and manufacturers of both biosimilars and originator products. ASBM, which has 16 Canadian members, is a member of the Canadian Biosimilars Working Group, has hosted and participated in a series of meetings with Health Canada and provincial health ministries on the topic of biosimilars and in 2017 conducted a survey of 403 Canadian prescribers of biologics.
Biosimilars are highly similar to the original biologic medicines but not identical, therefore they have not been considered “generic” drugs or appropriate for substitution for the original product without involvement of the prescribing health care provider.
Governments looking to control health costs and improve access to biologic therapies may turn to biosimilars as a tool. However, the announcement in B.C. is an example of “non-medical switching”- switching that results from or is driven by policy changes rather than the patient’s best medical interest. The controversial practice is often euphemistically referred to as a “transition” by proponents. While biosimilars are safe and effective products in their own right, no studies are required to demonstrate the safety and efficacy impacts of switching patients between originator and biosimilar. Canadian physicians have expressed serious concerns with third parties forcing non-medical biosimilar switching of patients who are stable and well-treated on their current medicine, effectively removing patient care from the physician’s authority.
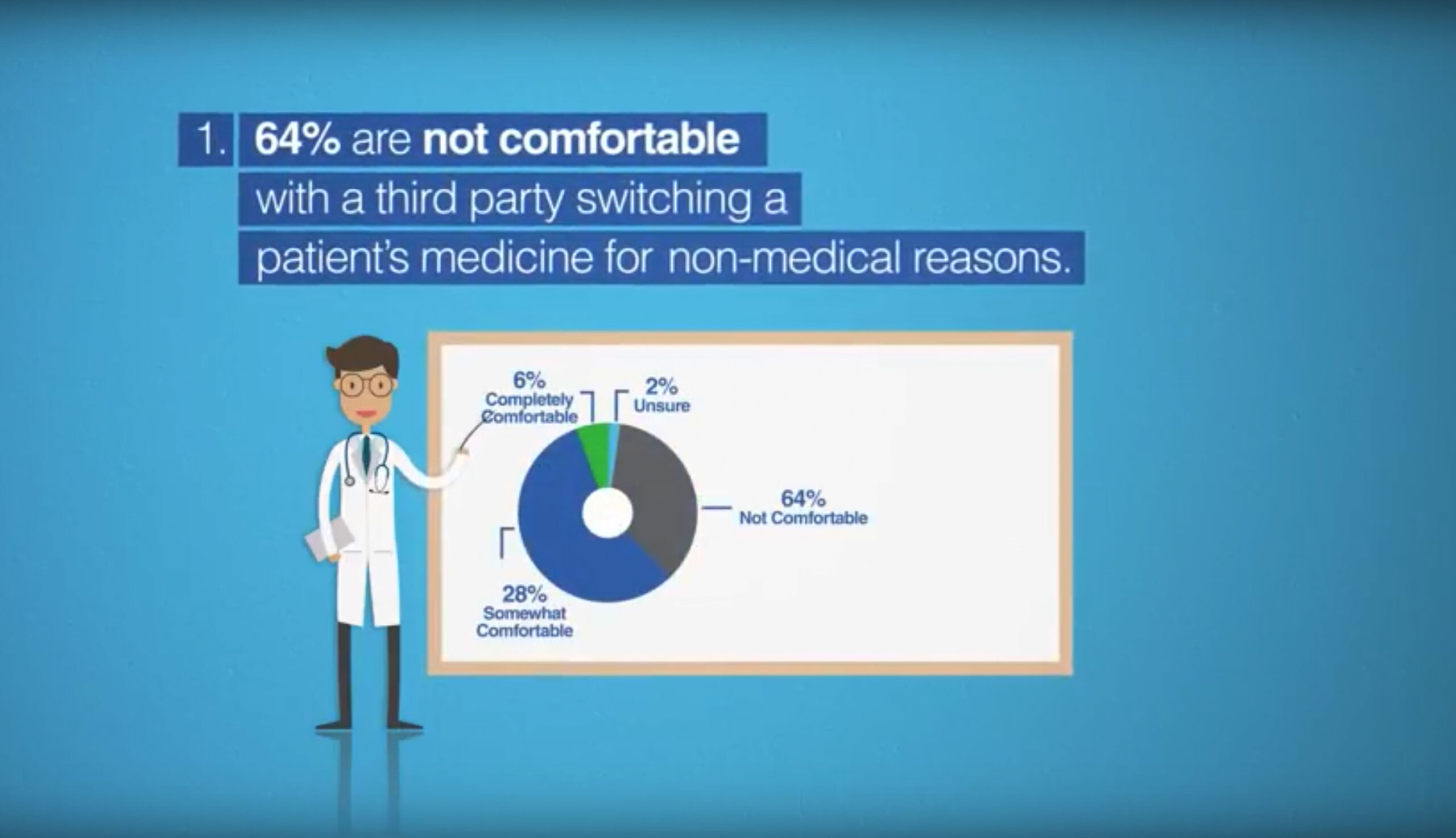
According to the 2017 Canadian prescriber survey:
- 64% were not comfortable with a third party switching a patient’s biologic medicine for non-medical (e.g. cost) reasons.
- 83% considered it “very important” or “critical” that prescribing physicians decide the most suitable biologic for their patients;
- 79% considered it “very important” or “critical” to have the authority to designate on a prescription for a biologic medicine “Dispense as Written” or “Do Not Substitute”;
- 82% of physicians support switching studies before a third party may automatically substitute a biologic.
Health Canada recommends that a decision to switch a patient to a biosimilar “should be made by the treating physician in consultation with the patient and taking into account available clinical evidence and any policies of the relevant jurisdiction.”
“Governments looking to expand access and trim health costs, such as B.C.’s, should view the legitimate concerns of health-care professionals and patients as an opportunity to provide education and increase choice and competition, as has been the successful approach in other countries,” said Michael Reilly, executive director of ASBM.
The B.C. government took this opportunity to announce it would begin to cover two new biologic medicines, both already covered in neighboring provinces. “Providing access to new biologic medicines is commendable and appropriate but linking access to the forced switching of patients and limiting physician choice is not acceptable,” Reilly added. “As many countries have shown, there are other ways to put patients first and realize savings without restricting medication choice. In Europe, for example, the vast majority of countries do not force well-treated patients to switch biologics, yet they have a robust and evolving biosimilar market. In light of physician, pharmacist and patient concerns, in the absence of any medical benefit, and with other ways to control costs, we join patients, physicians and pharmacists in opposing this approach and urging reconsideration.”
Contact: media@safebiologics.org