While all FDA-approved biosimilars are safe and effective, INTERCHANGEABLE BIOSIMILARS are biosimilars that have provided additional data to the FDA demonstrating that a patient can be switched between the biosimilar and the reference product and still expect the same result without additional risks. Under state laws, only these can be substituted by third parties such as insurance companies or pharmacy benefit managers (PBMs).
INTERCHANGEABLE BIOSIMILARS BUILD PHYSICIAN CONFIDENCE
While 89% of physicians believe biosimilars are safe and effective, treatment plans are not one-size-fits-all. Patients often try many safe and effective medicines before finding one that works best for them. For this reason, physicians are reluctant to switch patients’ medicine unnecessarily. 69% believe that the patient and physician, not a third party, should determine the most appropriate biologic medicine to use.
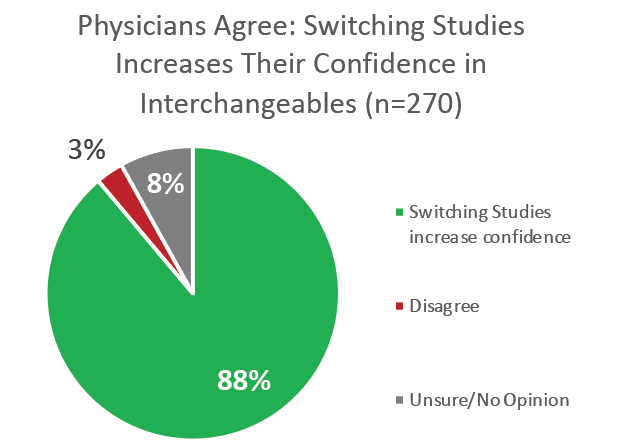
But the interchangeable biosimilar designation has proven successful in promoting confidence in automatic and third-party substitution among a majority of physicians: 57% said they’d be more likely to prescribe an interchangeable biosimilar; 59% said that the data required to earn an interchangeability designation makes them more comfortable with a pharmacy-level substitution of a biosimilar in place of the originator. Often the FDA asks for data includes switching studies, which 88% of physicians have said increases their confidence in biosimilar substitution.
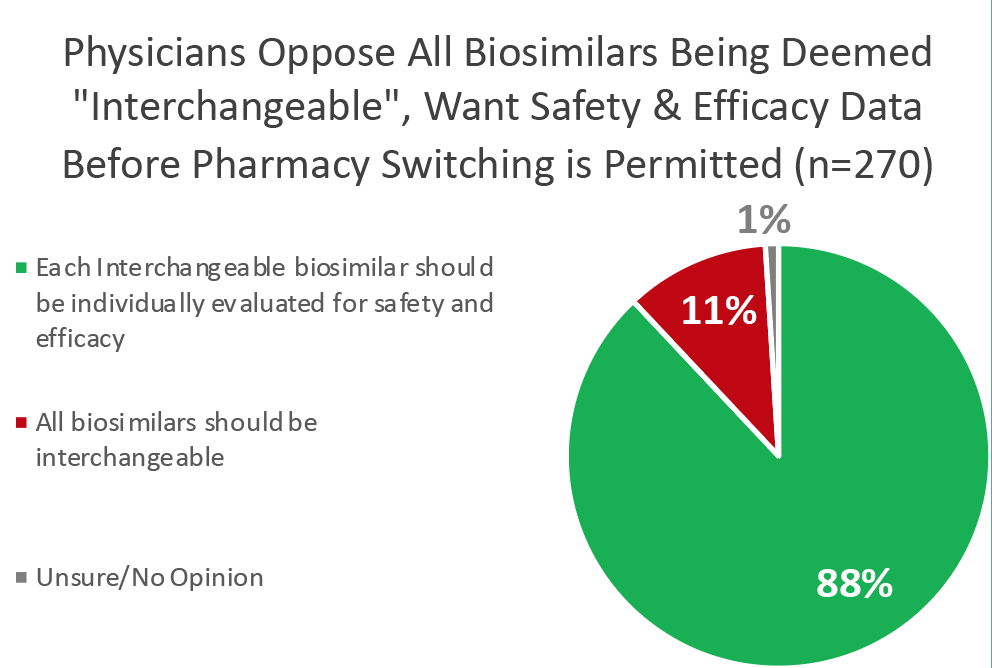
But some policymakers have suggested weakening these standards by declaring all biosimilars interchangeable (substitutable a the pharmacy by third parties such as insurance companies or PBMs), despite only 11% of physicians supporting this. Other proposals restrict the FDA’s ability to ask for switching studies.
ASBM RESOURCES ON INTERCHANGEABLE BIOSIMILARS
ASBM Letter Opposing S. 2305 “The Biosimilar Red Tape Elimination Act”
Fact Sheet: Why Physicians and Patients Oppose S. 2305 “The Biosimilar Red Tape Elimination Act”
ASBM Comments to FDA on Draft Guidance De-Emphasizing Switching Studies
U.S. Physician Survey (n=270) on Interchangeable Biosimilars
Press Release for U.S. Physician Survey on Interchangeable Biosimilars
Fact Sheet: What Do Physicians Think About Interchangeable Biosimilars?
Fact Sheet: Interchangeable Biosimilars: Comparing Europe and the U.S.
Fact Sheet: Policy Challenges for Interchangeable Biosimilars
ASBM Comments to Oregon Prescription Drug Affordability Review Board (PDAB) regarding the proposed substitution of non-interchangeable biosimilars
