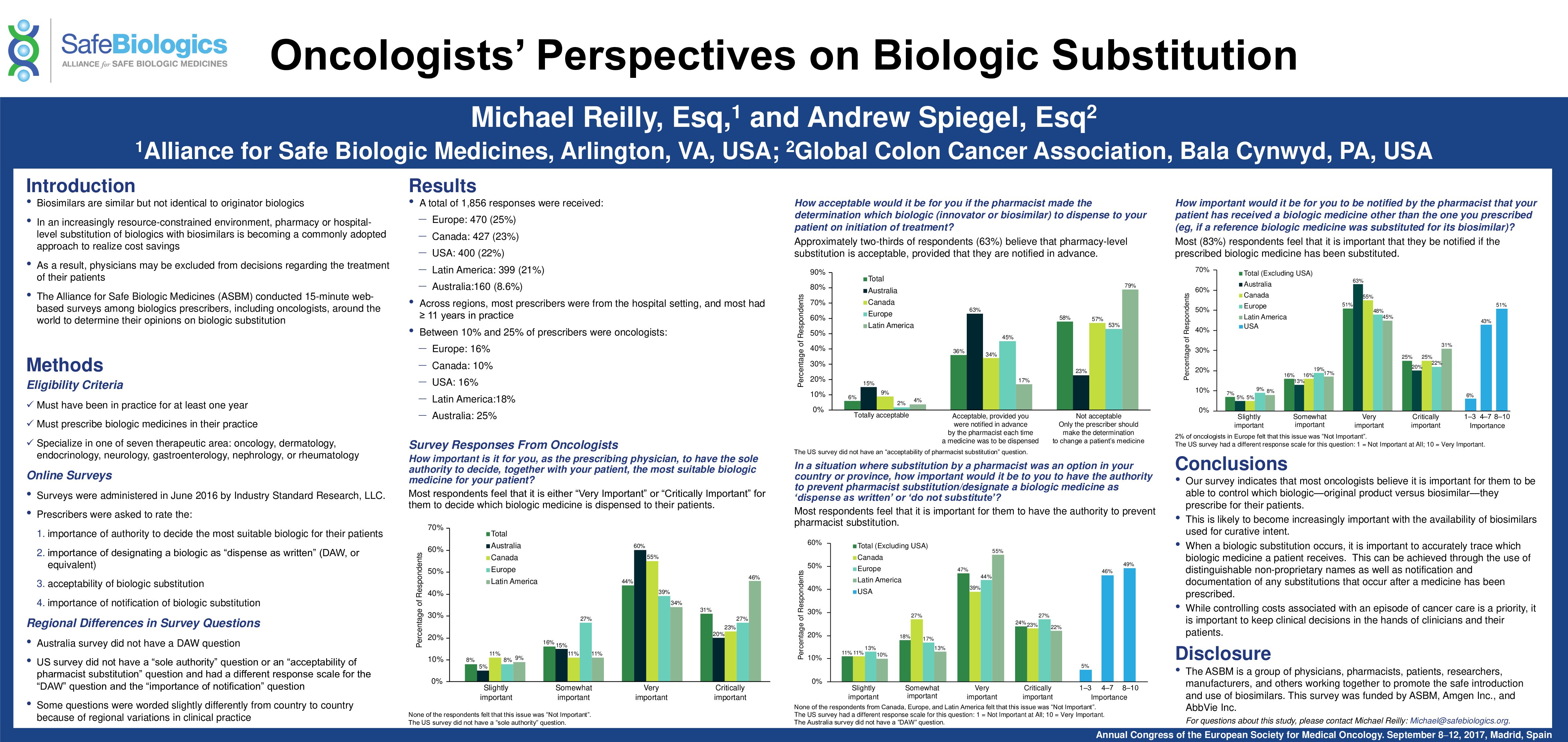
On September 10, 2017 ASBM presented a poster at the European Society of Medical Oncology (ESMO) 2017 Congress which presented perspectives of oncologists from 12 countries regarding biosimilar substitution. Responses were drawn from five recent ASBM surveys which gathered perspectives of more than 1,850 biologic prescribers in Australia, Canada, France, Germany, Italy, Spain, the United Kingdom, and the United States. ASBM Steering Committee Member Andrew Spiegel, head of the Global Colon Cancer Association, presented the poster at the conference.

Among the findings:
- Approximately two-thirds of respondents (63%) believe that pharmacy-level substitution is acceptable, provided that they are notified in advance.
- Most respondents feel that it is important for them to have the authority to prevent pharmacist substitution, with 81% of respondents considering this authority “very important” or “critical”.
- Most (83%) respondents feel that it is important that they be notified if the prescribed biologic medicine has been substituted.
View the poster here.