On March 6th in Ottawa, Ontario, ASBM hosted the third in a series of meetings between health regulators and other stakeholders from around the globe to discuss the international harmonization of biologic nomenclature and the importance of distinguishable naming. Representatives from Health Canada, the FDA, and WHO participated.

The meeting began with opening remarks by ASBM Executive Director Michael Reilly thanking the participants. He emphasized the shared support for global harmonization and for WHO leadership in that effort. Participants then introduced themselves; they included:
- Michael Reilly, Executive Director, ASBM
- Philip Schneider, MS FASHP FFIP; Advisory Board Chair, ASBM
- Madelaine Feldman, MD, FACR; Chair, ASBM
- Rafaella Balocco Mattavelli, INN Programme Lead, World Health Organization
- Anthony Ridgway, Health Canada
- Stephanie Hardy, Health Canada
- Andrew Spiegel, Global Colon Cancer Association
- Gail Attara, Gastrointestinal Society
- Ganive Bhinder, Better Pharmacare Coalition
- Laurie Proulx, Canadian Arthritis Patient Alliance
- Jaymee Maaghop, Canadian Cancer Survivor Network
- Christine Janus, International Association of Dermatology Patient Organizations
- Eric Lamoreaux, Canadian Patient Safety Institute
- Maureen Smith, Canadian Organization for Rare Disorders
- Angie Hamson, Patients for Patient Safety Canada
- Karen J. Kieley, Royal College of Physicians and Surgeons
- Caroline Herzberg, Canadian Dermatology Association
ASBM Advisory Board Chair Philip Schneider then presented a recap of the first two meetings, which were held in April and July of last year in Washington, DC. In these meetings, a strong consensus emerged on the importance of distinct naming and in favor of international harmonization. Health Canada in particular was a leading advocate of both.
Schneider then recounted several developments in the naming discussion that have occurred since the July meeting. First was the publication of the Scientific American whitepaper based on the April meeting, which may be read here. Second was ASBM’s participation in the 67th INN Consultation on October 13, 2018. Third was the publication of an article in the GaBI Journal emphasizing the benefits of the WHO’s Biological Qualifier (BQ) proposal for countries in the Middle East and North Africa region without robust pharmacovigilance systems of their own. The BQ would assign a 4-letter suffix to all biologics and biosimilars sharing an INN.
Dr. Schneider also shared objections to distinct naming- in particular to the WHO’s BQ proposal- that have been raised over the years, including that the system would be redundant, or might impede access, and proceeded to address them. For example, while advanced countries in Europe have robust pharmacovigilance systems, many developing nations do not. The Ministry of Health in the United Arab Emirates, Schneider noted, has expressed its support for the WHO’s BQ proposal, as have physicians in Latin America, 94% of whom consider it helpful in ensuring their patients receive the correct medicine.
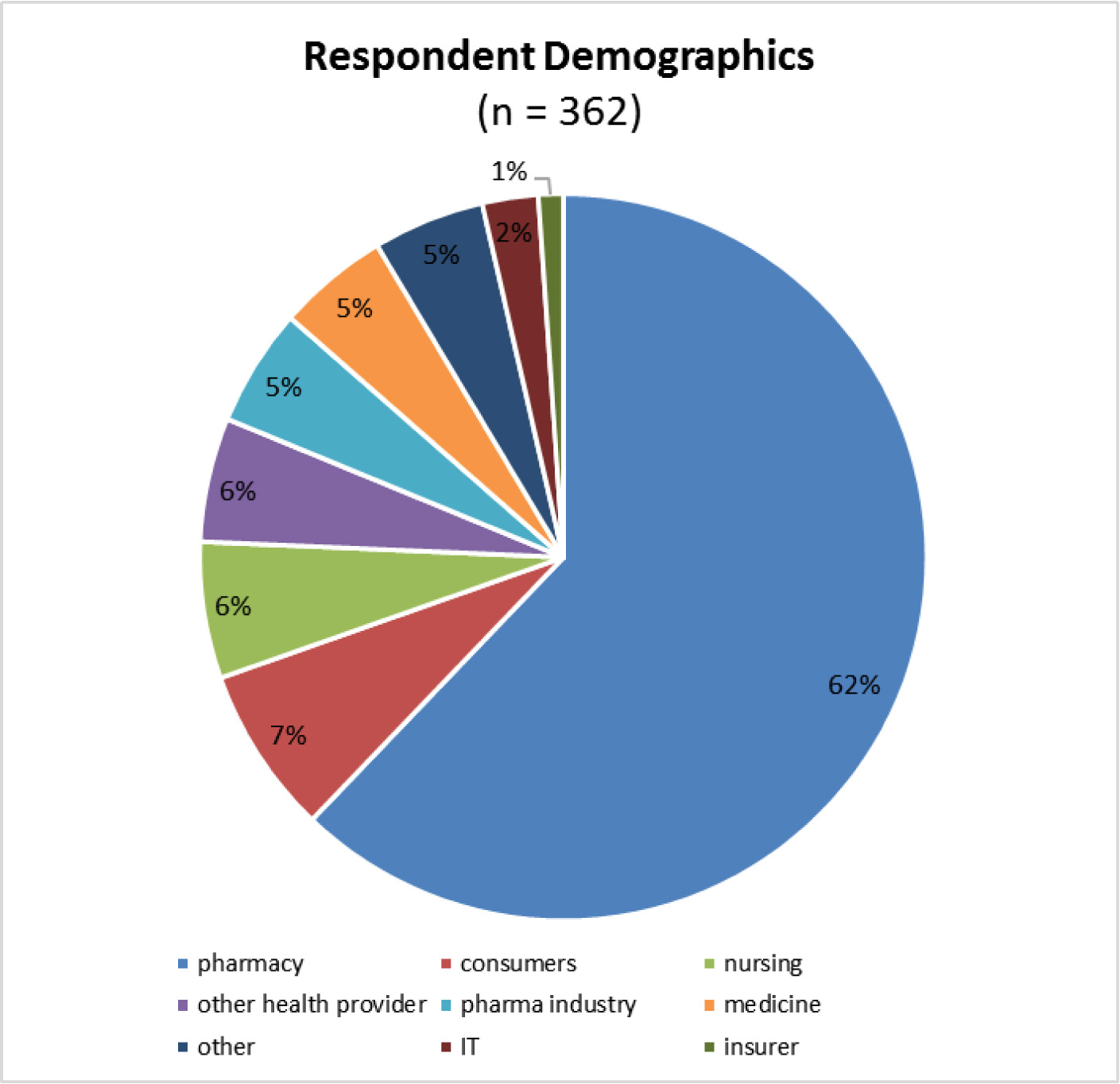
As Dr. Schneider observed, this consultation and the resulting policy fails to adequately consider not only the perspectives of patients, but that of physicians.
 For example, in a 2017 survey of 403 Canadian prescribers of biologics, ASBM found:
For example, in a 2017 survey of 403 Canadian prescribers of biologics, ASBM found:
- 68% of physicians supported Health Canada assigning unique, distinct names to all biologics and biosimilars.
- Only 1% of these physicians said they identify the medicine in the patient record using the DIN.
- 20% record only the product’s non-proprietary name in the patient record, not the brand name. This can result in inadvertent or inappropriate substitutions, as well as the physician not being aware of which among a number of products the patient will be dispensed at the pharmacy.
- When reporting adverse events, only 4% of physicians use the DIN.
- When reporting adverse events, 26% record only the product’s non-proprietary name. This can result in misattribution of an adverse event to the wrong product.
- Only 23% of physicians consistently record batch/lot number when reporting adverse events.

FDA official Lubna Merchant, who attended the first two meetings, joined the meeting via teleconference.




 Dr. Balocco also expressed hope that Canada’s decision would further underscore the urgent need for the WHO to take a leadership role on biologic naming and implement the BQ standard.
Dr. Balocco also expressed hope that Canada’s decision would further underscore the urgent need for the WHO to take a leadership role on biologic naming and implement the BQ standard.

