WHO INN Programme Lead Dr. Raffaella Balocco visits with Advisory Board Chair Philip Schneider (left) and Executive Director Michael Reilly (right) at ASBM’s booth.
From June 24th to June 26th, ASBM exhibited at the DIA 2019 Global Annual Meeting in San Diego, CA. ASBM was represented by Executive Director Michael Reilly and Advisory Board Chair Philip Schneider, who met with conference attendees to discuss ASBM’s work.
ASBM distributed literature on key biosimilar policy issues including: biosimilar basics, distinct naming, substitution and interchangeability, product labeling, indication extrapolation, and international harmonization of biologic nomenclature. Among the booth’s visitors was WHO INN Programme Lead Dr. Raffaella Balocco, who was a speaker at a DIA panel on pharmacovigilance.

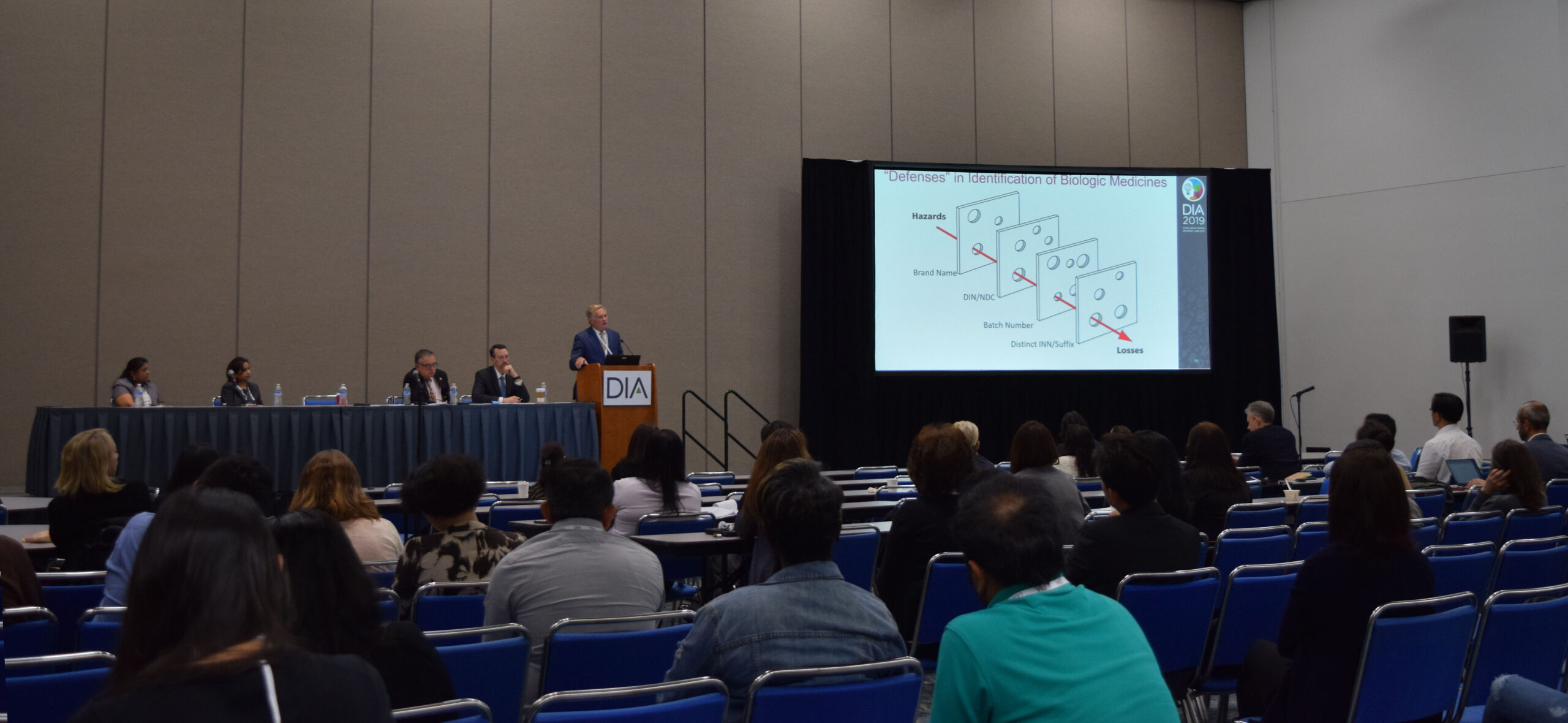
On Thursday, June 27th, Dr. Schneider participated in a session entitled “Successes and Challenges in Pharmacovigilance for Biologics and Biosimilars“. In his presentation, Schneider discussed the importance of redundancy in high reliability systems, with respect to clear product identification and biologic naming. Schneider noted that in Europe, where multiple biosimilars share a nonproprietary name with the originator biologic upon which they are based, roughly a third of adverse event reports for infliximab products do not identify the specific product responsible by its brand name.

A system of distinct nonproprietary naming (such a the suffix systems proposed by WHO and enacted by FDA) would add an additional safeguard and minimize the risks of such pharmacovigilance problems, Schneider explained. View Dr. Schneider’s presentation here.

Other presenters in the session included Kalindi Hapani, MPharm of APCER Life Sciences; Brian Edwards, DrMed, of ACRES, NDA Group; and Lubna Merchant, PharmD, MS, of FDA.